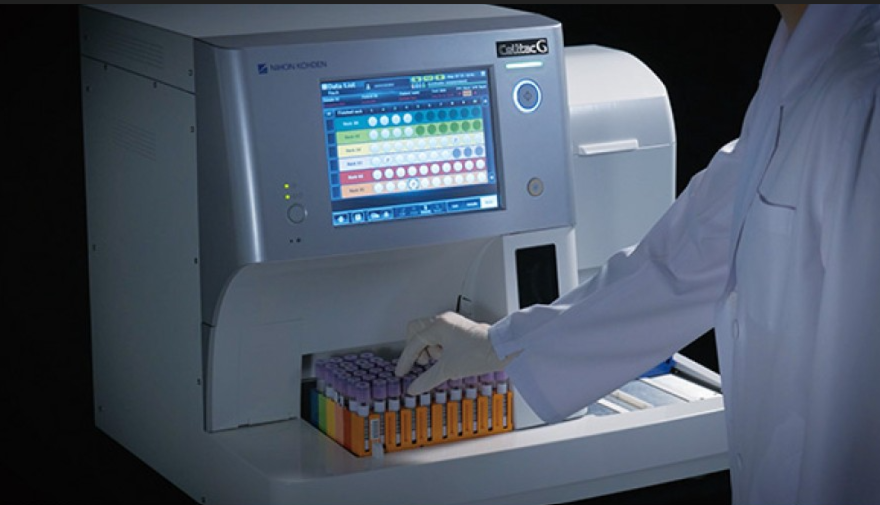
Services
Are you looking for information about a specific service or product? Please be aware that this website is still being translated, and some content may appear in its original language. We are working to complete the translations and appreciate your understanding.
You Purchase – We Care
Tailor-Made Service Packages for Reliable Healthcare Solutions
Flexible service plans designed for hospitals and medtech environments. From installation and training to preventive maintenance, rapid repairs, and compliance updates—our experts keep your systems running at peak performance so you can focus on patient care.
Standard
Essential Protection & Maintenance
- Technical support (phone or email)
- Preventive maintenance
- Electrical safety test
- Software-Update
- Maintenance report

Advanced
Extended Service coverage
- Everything in Standard, plus
- Loaner device for bridging
- Inspection of main components
- Repair (labour houtrs)
- Spare parts

Premium
Complete Care & Cost Control
- Everything in Advanced, plus:
- Less invoicing and booking
- Predictable costs
- All inclusive service
- (no extra spare parts costs)

Choose the level of cover that matches your clinical workload. From proactive maintenance and safety testing to on-site repair, loaner devices and all-inclusive contracts, our experts design a service plan around your hospital so your teams can focus on patients, not equipment. These service packages are our main examples. Certain components will be discussed and customized to suit your local needs
Product and Clinical Application Training
Achieving Excellence in Medical Equipment Handling
Our Product and Clinical Application Training is designed to help partners and external teams effectively use our products in real-world clinical settings. This training focuses on product features, best practices, and clinical applications to enhance user proficiency and patient outcomes.
Upcoming Events
Get in touch with your local expert
For over 70 years, Nihon Kohden has combined Japanese precision with advanced medical technology to support healthcare professionals across Europe. Our certified service teams ensure your equipment runs flawlessly—because patient care depends on it.
From consultation and demonstrations to installation, preventive maintenance, and rapid technical support, we provide end-to-end service solutions tailored to your hospital or clinic. With certified technicians, genuine spare parts, and continuous training, we guarantee maximum uptime, predictable costs, and long-term savings.
Partner with Nihon Kohden for a transparent, professional service relationship built on trust and excellence. When lives depend on technology, precision matters.

More Services
Product and software registration, safety data sheets, recycling passport and more
When it comes to regulatory compliance and product registrations, navigating this complex landscape can be daunting. At Nihon Kohden, we offer comprehensive support for various registrations to ensure that your Nihon Kohden medical devices comply with all necessary legal and safety requirements. Whether you need assistance with AED registration, software registration, safety data sheet creation.